INTRODUCTION
Periodontal diseases affect a significant portion of the adult population. Periodontitis is the primary cause of tooth loss in older adults.1 These problems also have been linked to systemic disease.2,3 The primary extrinsic etiologic factor for these problems is associated with bacteria.4 Consequently, treatment of inflammatory periodontal diseases is focused on the effective debridement of the subgingival environment, and reducing the bacterial load with personal daily oral hygiene and professional removal of bacterial biofilm and its by-products.
Clinicians have traditionally relied on clinical observation, tactile sensation, proper instrumentation technique, and experience to noninvasively treat shallow periodontal pockets. The speciality of periodontics has conducted years of research, debate, and clinical case care to analyze the benefit and the effectiveness of nonsurgical therapy. Clinical research has provided extensive support for the use of nonsurgical therapy to treat and maintain some forms of periodontal disease.5,6 As with any treatment modality, there are inherent limitations to effectively treat periodontitis on a predictable basis.
Limitations of Nonsurgical Therapy
Complete removal of subgingival bacterial biofilm and calculus remains a challenge.7-12 In an effort to overcome the various limitations of nonsurgical debridement, clinicians, scientific investigators, and dental manufacturers have expended effort and resources in the search for better treatment outcomes and the technologies to facilitate these improved treatment outcomes. In 1995, the American Academy of Periodontology suggested that the evidence-based treatment approach be established as a standard of care for the delivery of periodontal treatment.13 Many dental practitioners would agree that this philosophy of care improves clinical outcomes because care is based on documented evidence, rather than purely on empirical experience and anecdote. There is significant evidence in the dental literature describing the limitations of nonsurgical therapy. The literature has also demonstrated the inability to tactilely evaluate root surface deposits in periodontal pockets following debridement. This limits the clinician’s ability to fully instrument root surfaces in deeper periodontal pockets.7-9,11,14-17 Additional limitations in nonsurgical therapy include restricted access during instrumentation by various anatomic factors (eg, furcations, root grooves, concavities),18,19 other variations (eg, cemento-enamel projections,20,21 enamel pearls,22 the path of the cemento-enamel junction,23 cemental tears,24 and bifurcation ridges25) impede access and impede the ability to fulfill the goals of nonsurgical scaling and root planing. Due to these limitations, access to the primary etiology of periodontal diseases (ie, plaque and calculus) has been limited for all periodontal therapies.26
 |
 |
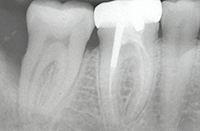
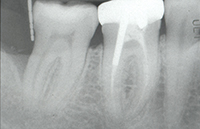
| Figure 1. Tooth No. 31 had a 12.0-mm distal probing depth and a one-wall distal intraosseous defect. | Figure 2. Tooth No. 31 clinically demonstrated an 8.0-mm distal probing depth 6 months following “blind” conventional nonsurgical debridement. |
Enhanced Visualization and Fiber-Optic Endoscopy
Noninvasive diagnostic imaging of tissues and body structures with the aid of magnetic resonance imaging and computed tomography is currently very common. Endoscopic and microscopic technologies have been employed in the surgical field of medicine for decades. Microsurgical techniques are not new in the field of periodontics. For years, magnification has been used to enhance visualization within the oral cavity, thereby increasing the precision of periodontal therapy. During microsurgical procedures, magnification allows the clinician to utilize smaller instruments that are less traumatic. Fiber-optic technology has also facilitated this increase in visual magnification and treatment precision. Historically, visual access to the subgingival root surface could only be attained through a periodontal flap procedure or extraction of the tooth. Now clinicians can deliver minuscule fiber-optic probes into the periodontal pocket to gain visual access.
Fiber-optic periodontal endoscopes used during instrumentation may improve scaling and root planing efficacy in deeper pockets. Advancements in fiber-optic technology, coupled with modifications of the periodontal armamentarium (eg, curets, periodontal probe, ultrasonic scaler) have led to the development of a technology that allows the clinician direct visual access of the subgingival root surface within the periodontal pocket.27
Fiber-optic endoscopes contain bundles of thin glass fibers that use the principle of total internal reflection to transmit light to and from the organ being viewed and to transmit almost 100% of the light entering one end to the other. Fiber-optic endoscopes are delicate and expensive instruments. The fibers are made of a special glass, and each fiber is coated with a layer of glass with a different refractive index. Additionally, the orientation of the fibers in a bundle used for endoscopy must be “coherent” in spatial orientation for its full length.
Dental Endoscope
The Dental Endoscope, originally developed by Dentalview Incorporated and currently available from Perioscopy Incorporated, consists of a miniature fiber-optic cable that contains a 10,000-pixel scope that can magnify an image from 24x to 48x. The 0.85 mm diameter fiber-optic endoscope incorporates an integrated light source for subgingival illumination. This system incorporates the use of disposable sheaths for the fiber-optic cable and a water system to insuflate the pocket and provide lavage of the operative field. The fiber-optic image is captured through a charged coupling device camera and digital processor. The operative field is viewed on a flat panel video display.28
 |
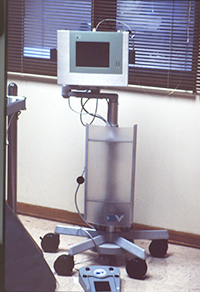 |
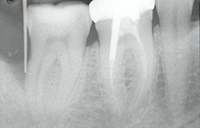
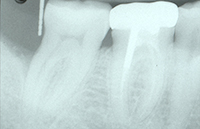
| Figure 3. Tooth No. 31 with an 8.0-mm distal probing depth (with no bleeding on probing) and one wall distal intraosseous defect. Note increased radiopacity of defect wall. | Figure 4. The periodontal endoscope. |
 |
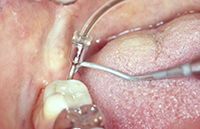 |
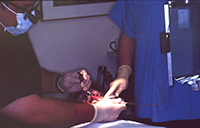
| Figure 5. Periodontal endoscope in clinical use. | Figure 6. Periodontal endoscope explorer in use on the distal of tooth No. 31. |
 |
 |
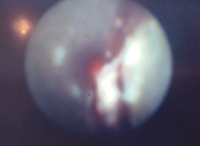
| Figure 7. Endoscopic monitor image. Subgingival bacterial biofilm and subgingival calculus present approximately 6.0 mm to 7.0 mm into the “pocket.” |
Figure 8. Burnished subgingival calculus deposit. |
 |
| Figure 9. Endoscopic monitor image. Clean subgingival root surface following definitive endoscopic root debridement. |
Clinical Trials and Human Histology
Recent clinical trials and human histologic studies have demonstrated the effectiveness of nonsurgical debridement with the aid of the periodontal endoscope. Wilson et al,29,30 in 2008, hypothesized that the endoscope may increase the diagnostic value of gingival redness. Gingival redness is among the cardinal signs of inflammation and a standard component of virtually all clinical indices used to assess the severity of gingival inflammation.31-34 However, this parameter measured supragingivally does not have a high predictive value for the progression of periodontitis.35 Wilson et al29,30 examined 26 patients with moderate to severe periodontitis with the periodontal endoscope. The findings showed a statistically significant relationship between calculus covered with biofilm and inflammation, ginigival redness, in the pocket wall. More than half of the cases of the inflammation were associated with bacterial biofilm and calculus, as opposed to bacterial biofilm alone. Only subgingival calculus had a statistically significant relationship to the positive traditional gingival index. They29,30 showed that deposits of subgingival calculus covered with biofilm were directly related to more than 60% of pocket wall inflammation as measured by increased redness of the pocket epithelium. This was in comparison to biofilm alone, which was present in more than 30% of the sites where inflammation was observed. They29,30 concluded that calculus may be a factor in subgingival inflammation. Because it has been shown that sterilized calculus does not create inflammation,36 it can be hypothesized that calculus, in some way, enhances the inflammatory effect of the biofilm observed. They29 also went on to say that, “The current study found that subgingival inflammation was unrelated to any of the traditional measures of inflammation, including gingival inflammation (traditional gingival index) and the presence of bleeding on probing. However, deeper probing depths were related to subgingival inflammatory changes as viewed through the endoscope.”29
In the companion study, again in humans, calculus was removed with the aid of an endoscope. This study showed no histologic signs of chronic inflammation at 6 months following a single episode of closed, subgingival scaling and root planing using the endoscope. Thus, “complete removal of subgingival deposits of bacterial biofilm and by-products, including calculus, as defined by the use of an endoscope, may be appropriate if chronic inflammation is a problem.”30
In 2009, Mellonig et al37 reported on a 4-subject case report using conventional blind nonsurgical debridement and the application of an enamel matrix derivative (EMD). Enamel matrix derivative is a composite of proteins that has been demonstrated histologically to work as an adjunct to periodontal regenerative surgical therapy. The purpose of this study was to evaluate the clinical and histologic effects of EMD as an adjunct to scaling and root planing. The 4 subject patients had all been diagnosed with severe chronic periodontitis and were scheduled to receive complete dentures. This study recorded probing depth and clinical attachment levels for each patient. Conventional hand and ultrasonic root surface instrumentation was completed and not limitied by time. The root was indexed for later histologic examination, and EMD was expressed into the root surface. Plaque control was accomplished for the patient every 2 weeks. Six months after treatment, probing depth and clinical attachment levels were again recorded, and the teeth were removed en bloc. The samples were then prepared and examined histologically. Probing depth reduction and clinical attachment level gains were obtained in three fourths of the specimens. Three of the 4 specimens when analyzed histologically demonstrated new cementum, bone, periodontal ligament, and connective tissue attachment coronal to the notch. In one specimen, the gingival margin had receded below the notch. The investigators37 reported that, “These results were unexpected and may represent an aberration. However, the substantial reduction in deep probing depths and clinical attachment level gain in 3 of 4 specimens, in addition to the histologic findings of new cementum, new bone, a new periodontal ligament, and a new connective tissue attachment, suggest that EMD may be useful as an adjunct to scaling and root planing in single-rooted teeth.”37
This case report will present the successful treatment of localized severe chronic periodontitis.
 |
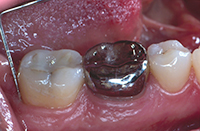 |
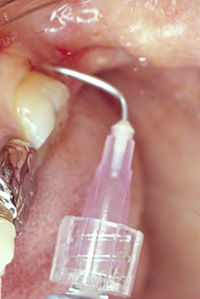
| Figure 10. Emdogain (Straumann) enamel matrix derivative (EMD) placed subgingivally following smear layer removal with PrefGel (Straumann) a neutral pH buffered, water soluble ethylenediamine tetra-acetic acid. | Figure 11. Tooth No. 31 with a 3.0-mm distal probing depth at 14 months following secondary (endoscopic debridement and EMD regeneration) therapy. |
 |
 |
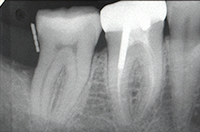
| Figure 12. Tooth No. 31 had a 3.0-mm distal probing depth and early radiographic defect fill. (Note: Compare to Figure 1 and Figure 3.) | Figure 13. At a 6 year follow-up, tooth No. 31 had a 3.0-mm distal probing depth and continuing radiographic defect fill. (Note: Compare to Figure 3 and Figure 12.) |
 |
| Figure 14. At 8.5 year follow-up, tooth No. 31 had a 3.0 mm distal probing depth and continuing radiographic defect fill. (Note: Compare to Figures 1, 3, 12, and 13.) |
CASE REPORT
Diagnosis and Treatment Planning
A 43-year-old white female was referred by her husband, a current patient. She presented with a chief complaint of, “I have a pocket behind my lower right last molar…and I don’t want surgery.” The patient’s health history was not remarkable. Her dental history and patient interview revealed an ongoing cigarette habit (one quarter pack per day for the last 23 years). Her interview also revealed a traumatic dental experience, at the age of 19 years, associated with the surgical removal of an impacted third molar. During the last 5 years, adult prophylaxis had been completed at 6-month intervals. The historical clinical records that accompanied the patient documented a 10-mm probing depth with bleeding on probing present on the distal aspect of tooth No. 31. This defect had been present for the one year period prior to the patient’s referral.
Initial examination of the patient revealed a 10-mm distobuccal and 12-mm distolingual probing depth with bleeding on probing and no recession on the distal aspect of tooth No. 31. There was also an incipient buccal furcation invasion with an accompanying 9 mm probing depth and no recession on the buccal of tooth No. 31. The tooth had no mobility (Figure 1). The guarded prognosis for tooth No. 31 and initial treatment recommendations were discussed at length with the patient.
The patient would only commit to a more frequent supportive periodontal therapy (SPT) schedule. For the first 6 months, SPT was completed on a 3-month interval. This treatment approach was followed to allay the patient’s anxiety, to build patient trust, and to provide an opportunity to help the patient improve daily oral hygiene.
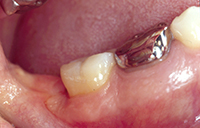
After the first 6 months of patient care, initial treatment recommendations and alternatives were discussed a second time with the patient. The patient elected conventional “blind” nonsurgical debridement of tooth No. 31 under local anesthesia. At the patient’s next 3-month SPT appointment, it was determined that this treatment had resulted in a 4-mm reduction in distal probing depths and a 6-mm reduction in buccal probing depth with no bleeding on probing in either area (Figures 2 and 3). At this SPT appointment, secondary treatment recommendations and alternatives were discussed with the patient. She elected endoscopic nonsurgical debridement and Emogain (Straumann) EMD regeneration for the residual defect on the distal of tooth No. 31 (Figure 4).
Secondary Therapy
Under profound local anesthesia (1.8 mL lidocaine 2% with 1:100,000 epinephrine), the periodontal endoscope was inserted to gain visual access of the subgingival root surface (Figure 5). A manually tuned magnetostrictive ultrasonic unit was used with the periodontal endoscope (using a 2-handed technique) to debride the root surface. The root surface was instrumented (Figures 6 and 7) and visually assessed as clean with the periodontal endoscope (Figures 8 and 9). The smear layer on the root surface was removed using a 2-minute application of PrefGel (Straumann), a neutrally pH buffered, water soluble ethylenediamine tetra-acetic acid gel. The PrefGel was washed away with sterile water in a 50 cc syringe. Emdogain EMD gel was applied to the root surface (Figure 10) using the prepackaged syringe; starting at the base of the defect and expressing the EMD gel as the syringe was drawn coronally. The EMD gel was left undisturbed for 2 minutes. Postoperative instructions were reviewed with the patient. The patient was instructed to use over-the-counter ibuprofen for discomfort following the label instructions.
| Table. Tooth No. 31 | ||||||||||||||||||||||||||||||||
|
Follow-Up and Supportive Periodontal Therapy
No specific follow-up appointments were scheduled following endoscopic debridement, as they would be scheduled following conventional open-flap debridement. Follow-up was accomplished at the patient’s regularly scheduled SPT appointments where instrumentation was guided by periodontal examination. Six months following endoscopic secondary therapy (at the second 3-month SPT appointment), it was determined the treatment had resulted in a further 2-mm distal probing depth reduction.
SPT was continued at a 3-month interval. At the 14-month appointment, an additional 2 mm distal probing depth reduction was noted (Figures 11 and 12). The clinical parameters on tooth No. 31 were now 2 to 3 mm probing depth with no bleeding on probing and no clinical access to either furcation entrance.
The clinical status of tooth No. 31 has remained improved and stable for the last 8 years and 4 months (Figures 13 and 14) (Table).
CONCLUSION
The treatment of localized severe chronic periodontitis, as presented in this case report article, incorporated the use of the dental endoscope to facilitate subgingival visualization during nonsurgical instrumentation and a porcine derived EMD for the regeneration of the periodontal attachment apparatus. The treatment resulted in significant probing depth reduction without recession. Following successful treatment, supportive periodontal care occurred over a 9-year period, during which radiographic osseous defect improvement was documented.
References
- Fox CH, Jette AM, McGuire SM, Feldman HA, et al. Periodontal disease among New England elders. J Periodontol. 1994;65:676-684.
- Mattila KJ, Pussinen PJ, Paju S. Dental infections and cardiovascular diseases: a review. J Periodontol. 2005;76(suppl 11):2085-2088.
- Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: An epidemiologic perspective. Ann Periodontol. 2001;6:99-112.
- Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134-144.
- Badersten A, Nilvéus R, Egelberg J. Effect of nonsurgical periodontal therapy. I. Moderately advanced periodontitis. J Clin Periodontol. 1981;8:57-72.
- Pihlstrom BL, McHugh RB, Oliphant TH, et al. Comparison of surgical and nonsurgical treatment of periodontal disease. A review of current studies and additional results after 6 1/2 years. J Clin Periodontol. 1983;10:524-541.
- Rabbani GM, Ash MM Jr, Caffesse RG. The effectiveness of subgingival scaling and root planing in calculus removal. J Periodontol. 1981;52:119-123.
- Caffesse RG, Sweeney PL, Smith BA. Scaling and root planing with and without periodontal flap surgery. J Clin Periodontol. 1986;13:205-210.
- Buchanan SA, Robertson PB. Calculus removal by scaling/root planing with and without surgical access. J Periodontol. 1987;58:159-163.
- Fleischer HC, Mellonig JT, Brayer WK, et al. Scaling and root planing efficacy in multirooted teeth. J Periodontol. 1989;60:402-409.
- Sherman PR, Hutchens LH Jr, Jewson LG, et al. The effectiveness of subgingival scaling and root planing. I. Clinical detection of residual calculus. J Periodontol. 1990;61:3-8.
- Caton JG, Zander HA. The attachment between tooth and gingival tissues after periodic root planing and soft tissue curettage. J Periodontol. 1979;50:462-466.
- McGuire MK, Newman MG. Evidenced-based periodontal treatment. I. A strategy for clinical decisions. Int J Periodontics Restorative Dent. 1995;15:70-83.
- Waerhaug J. Healing of the dento-epithelial junction following subgingival plaque control. II: As observed on extracted teeth. J Periodontol. 1978;49:119-134.
- Jones WA, O’Leary TJ. The effectiveness of in vivo root planing in removing bacterial endotoxin from the roots of periodontally involved teeth. J Periodontol. 1978;49:337-342.
- Stambaugh RV, Dragoo M, Smith DM, et al. The limits of subgingival scaling. Int J Periodontics Restorative Dent. 1981;1:30-41.
- Rateitschak-Plüss EM, Schwarz JP, Guggenheim R, et al. Non-surgical periodontal treatment: where are the limits? An SEM study. J Clin Periodontol. 1992;19:240-244.
- Gher ME Jr, Vernino AR. Root anatomy: a local factor in inflammatory periodontal disease. Int J Periodontics Restorative Dent. 1981;1:52-63.
- Withers JA, Brunsvold MA, Killoy WJ, et al. The relationship of palato-gingival grooves to localized periodontal disease. J Periodontol. 1981;52:41-44.
- Masters DH, Hoskins SW. Projections of cervical enamel in molar furcations. Periodontics. 1964;35:49-53.
- Hou GL, Tsai CC. Cervical enamel projection and intermediate bifurcational ridge correlated with molar furcation involvement. J Periodontol. 1997;68:687-693.
- Gher ME, Vernino AR. Root morphology—clinical significance in pathogenesis and treatment of periodontal disease. J Am Dent Assoc. 1980;101:627-633.
- Schroeder HE, Scherle WF. Cemento-enamel junction—revisited. J Periodontal Res. 1988;23:53-59.
- Moskow BS. Calculus attachment in cemental separations. J Periodontol. 1969;40:125-130.
- Löe H, Theilade E, Jensen SB, et al. Experimental gingivitis in man. 3. Influence of antibiotics on gingival plaque development. J Periodontal Res. 1967;2:282-289.
- Stambaugh RV, Myers G, Ebling W, et al. Endoscopic visualization of submarginal gingival root surfaces. J Dent Res. 2000;79(Special Issue):600 (Abstract 3656).
- Kwan JY. Enhanced periodontal debridement with the use of micro ultrasonic, periodontal endoscopy. J Calif Dent Assoc. 2005;33:241-248.
- Stambaugh RV, Myers G, Ebling W, et al. Endoscopic visualization of the submarginal gingiva dental sulcus and tooth root surfaces. J Periodontol. 2002;73:374-382.
- Wilson TG, Harrel SK, Nunn ME, et al. The relationship between the presence of tooth-borne subgingival deposits and inflammation found with a dental endoscope. J Periodontol. 2008;79:2029-2035.
- Wilson TG Jr, Carnio J, Schenk R, et al. Absence of histologic signs of chronic inflammation following closed subgingival scaling and root planing using the dental endoscope: human biopsies—a pilot study. J Periodontol. 2008;79:2036-2041.
- O’Leary T, Givson WJ, Shannon I, et al. A screening examination for detection of gingival and periodontal breakdown and local irritants. Periodontics. 1963;1:167-174.
- Loesche WJ. Clinical and microbiological aspects of chemotherapeutic agents used according to the specific plaque hypothesis. J Dent Res. 1979;58:2404-2412.
- Lobene RR, Weatherford T, Ross NM, et al. A modified gingival index for use in clinical trials. Clin Prev Dent. 1986;8:3-6.
- Halazonetis TD, Haffajee AD, Socransky SS. Relationship of clinical parameters to attachment loss in subsets of subjects with destructive periodontal diseases. J Clin Periodontol. 1989;16:563-568.
- Page RC, Beck JD. Risk assessment for periodontal diseases. Int Dent J. 1997;47:61-87.
- Allen DL, Kerr DA. Tissue response in the guinea pig to sterile and non-sterile calculus. J Periodontol. 1965;36:121-126.
- Mellonig JT, Valderrama P, Gregory HJ, et al. Clinical and histologic evaluation of non-surgical periodontal therapy with enamel matrix derivative: a report of four cases. J Periodontol. 2009;80:1534-1540.
Dr. Young graduated from the Indiana University School of Dentistry. After completing an Advanced Education in General Dentistry (AEGD) at the Irwin Army Hospital in Fort Riley, Kan, Dr. Young practiced general dentistry for 5 years. He completed his residency in periodontology at the University of Michigan. Dr. Young maintains a private practice limited to periodontics in Des Moines, Iowa. His practice has an emphasis in aesthetic periodontal regeneration, cosmetic periodontal reconstruction, and dental implants. He can be reached at (515) 224-1771 or at ryoungdsm@aol.com.
Disclosure: Dr. Young reports no disclosures.











